Protien in Soy Protien in Beef
Introduction
In contempo years, excessive intake of meat and meat products has been suggested to exist associated with some metabolic disorders (Tilman and Clark, 2014). Specifically, N-nitroso-compounds and heterocyclic amines, which were formed during cooking of red meat at high temperatures, could exist disquisitional factors for an elevated adventure of mortality of colorectal cancer (Pan et al., 2012; Bastide et al., 2015). Still, it is the fact that meat has many biological functions in terms of highly bioavailable nutrients, including essential amino acids, heme iron, and vitamins (Pereira and Vicente, 2013).
Nutrient is a major factor that tin can shape gut microbiota (Subramanian et al., 2014). The gastrointestinal tract and residing bacteria accept been shown to play a crucial role in extracting and metabolizing dietary ingredients (Muegge et al., 2011; Tyakht et al., 2012; Tang et al., 2013). There is well-nigh 12–eighteen yard of protein entering into homo colon every day, consisting of residuum dietary proteins and endogenous enzymes secreted in the small intestine (Scott et al., 2013). Approximately x% of ingested proteins tin can reach the colon, which is dependent on the type and amount of protein consumed (Cummings, 1997). The residual dietary proteins and endogenous enzymes are the primary source of nitrogen for the growth of gut microbiota (Cummings and MacFarlane, 1991). Amino acids would become energy source in the distal colon (Hamer et al., 2012). Contempo studies indicated that the metabolites derived from gut microbiota may have a certain touch on host wellness, for example, short chain fat acids, especially butyrate, can be served as energy for host tissues (Flint et al., 2015). On the other hand, lipopolysaccharide (LPS), an endotoxin, tin can enter into the circulation and be leap to lipopolysaccharide-binding protein (LBP) in liver (Weiss, 2003; Zhao, 2013). The LPS-LBP complex further binds to CD14 receptor, which mediates the activation of macrophages to produce inflammatory cytokines (Lukkari et al., 1999).
Dietary intake influences the construction and activity of the trillions of microorganisms residing in the human gut (Wu et al., 2011; Muegge et al., 2011; David et al., 2014). For example, enterotypes were strongly associated with long-term diets, particularly protein and animal fatty (Bacteroides) vs. carbohydrates (Prevotella; Wu et al., 2011). Gut microbiota and their metabolites showed differences later on intake of casein and plant proteins (Geypens et al., 1997; Mean solar day, 2013; Rist et al., 2014), however, few data are bachelor on how meat proteins affect gut microbiota and their metabolic activities. Rats and humans had similar gut bacterial communities, and thus rats are used equally models to report the linkage betwixt dietary proteins and gut microbiota (Ley et al., 2006). Our contempo study showed that the rats fed with meat proteins have significantly different structure of gut microbiota in caecum from those fed with soy protein (Zhu et al., 2015). Yet, it remains unknown how gut microbiota and metabolites in colon answer to different dietary proteins. In this study, we fed growing rats with casein, soy, beef and craven proteins for 90 days and characterized colonic metabolites by using (Tilman and Clark, 2014) H NMR spectroscopy and gut microbiota past sequencing the V4-V5 region of 16S ribosomal RNA. Meanwhile, the levels of LBP mRNA/protein and CD14 mRNA/protein were measured to evaluate the bacterial endotoxin load to host. The association between colonic leaner and metabolites, and its significance for host wellness were discussed.
Materials and Methods
Animals and Samples
Four-calendar week-onetime male Sprague-Dawley rats (117 ± 10 g) were purchased from Zhejiang Experimental Animal Center (Zhejiang, Red china, SCXK9 <Zhejiang> 2008-00) and housed in a specific pathogen-free animal center (SYXK <Jiangsu> 2011-0037). Later 7-day acclimatization (poly peptide source: casein), the rats were assigned randomly to iv formulated diets with casein, and proteins from beef, craven and soy (north = 8 each group). Casein is the sole protein in standard rat diets recommended by the American Establish of Nutrition, and thus we gear up the casein grouping as the control. The formulated diets were prepared as described previously (Zhu et al., 2015). The animals were maintained individually in plastic ventilated cages and given water and diets ad libitum in a temperature and humidity (twenty.0 ± 0.5°C, 60 ± x%) controlled room with a 12 h light/dark bicycle. Experimental protocol involving animals was reviewed and approved by the Ethical Commission of Experimental Animal Center of Nanjing Agricultural University. All experiments were performed in accordance with the relevant guidelines and regulations of the Upstanding Committee of Experimental Animate being Center of Nanjing Agricultural University.
Subsequently xc-day feeding, all rats were sacrificed after 4 h fasting. The distal colonic contents were collected and transferred to two eppendorf tubes, then immediately frozen in liquid nitrogen and stored at −80°C for metabolomic and microbiota analyses.
Microbiota and Metabolomic Analysis
Microbiota analysis was referred to our previous study (Zhu et al., 2015). Briefly, the caecal contents were collected, frozen in liquid nitrogen, and stored at −80°C before being analyzed. DNA was extracted from each sample using the QIAamp DNA Stool Mini Kit (NO. 51504, Qiagen, Federal republic of germany) according to the manufacturer'due south protocol. The 16S ribosomal RNA (rRNA) gene from caecal contents was amplified with universal primers: F515 (5′-GTGCCAGCMGCCGCGG-3′) and R907 (five′-CCGTCAATTCMTTTRAGTTT-three′). The V4-V5 hypervariable region that is universal for near all bacterial taxa was applied for amplification. Purified amplicons were sequenced under the MiSeq platform (Illumina, San Diego, California, United states) according to the standard protocols in a commercial company (Shanghai Majorbio Bio-Pharm Technology Co., Ltd, Shanghai, Mainland china).
Metabolomic analysis was performed as follows: (1) 1.5 mL of ice-cold h2o was mixed with 300 mg of colonic samples, vortexed vigorously for i min, and sonicated at 4°C for 10 min. Then the samples were subjected to vortex shaking at 13,000 rpm for 30 min at iv°C and 450 μL of the supernatant was advisedly transferred to a fresh eppendorf tube, and 50 μL of a standard buffer solution (ACDSS, Anachro Certified DSS Standard Solution, 4.136 mM) was added and vortexed vigorously for 10 southward. The mixture was subjected to vortex shaking at 13,000 rpm for twenty min and a 480 μL of the supernatant was transferred to a NMR tube for subsequent NMR analysis. 1H-NMR spectra were obtained at 298 K under a Bruker AV Three 600 MHz spectrometer (operating at 1H frequency of 600.xiii MHz, Bruker Biospin, Frg) equipped with an inverse cyrogenic probe. A total of 32 scans were collected into 32 k data points for each spectrum with a spectral width of 8,000 Hz. Water presaturation for ane s along with the recycle delay was applied for solvent indicate suppression. All 1H-NMR spectra were processed and analyzed using the Chenomx NMR Suite Professional software package (version 7.vii, Chenomx Inc., Edmonton, Canada). Qualitative and quantitative analyses of 1H-NMR spectra were performed past manually plumbing fixtures spectral signatures from an internal database of Chenomx to each spectrum. The ACDSS was used as internal standard for chemical shift referencing (0 ppm) and quantification.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)-Based mRNA Assay
A semi-quantitative RT-PCR analysis was used to estimate the mRNA levels of LBP and CD14 in liver samples. Full RNA was isolated from liver samples using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Nihon) according to the manufacture'south protocol. Total RNA was quantified by a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, Delaware, United states of america) at 260/230 and 260/280 nm. Then 400 ng RNA was reversely transcribed into ten μL cDNA by using the PrimeScriptTM RT Chief Mix (TaKaRa, Japan) and the Peltier Thermal Cycler 200 (MJ Enquiry, Watertown, MA, USA). The cDNA was dissolved in RNase-gratuitous h2o and stored at −xx°C.
The two-stride qRT-PCR reactions were performed in triplicate on 96-well plates using a 7500 Existent-time PCR organization (Applied Biosysytems, Foster, CA) with the SYBR® Premix Ex TaqTM (TaKaRa, Ostu, Nihon). LBP (Lukkari et al., 1999), CD14 (Järveläinen et al., 1997) and β-actin primer sequences were synthesized by Sangon Biotech (Shanghai, People's republic of china). These primer sequences were listed in Table one. The concentrations of template and primers, the efficiency and consistency of LBP, CD14, and β-actin amplification were evaluated past a relative standard curve by echelon dilution (1:i–i:625). The reaction solution (xx μL) contains ten μL SYBR® Premix Ex Taq, 0.four μL PCR forward primer (10 μM) and 0.four μL PCR reserve primer (10 μM), 0.4 ROX reference dye II, 2 μL cDNA and half-dozen.8 μL dHiiO. Cycling conditions were every bit follows: xxx south for denaturation at 95°C, xl cycles of five s at 95°C and 34 s at 60°C for denaturation, followed by triple alternations between 95 and sixty°C for melting bend analysis to verify the specificity of a single amplification. Fold changes of LBP and CD14 expression were calculated by the two−ΔΔCt method normalized to β-actin, setting soy protein group every bit the control.

Table ane. Primers used for qRT-PCR
Western Blotting
Liver protein was extracted using a commercial poly peptide extraction kit (Thermo Pierce, NO. 78510). Whole poly peptide was quantified with an Enhanced BCA Poly peptide Assay Kit (Biyuntian, China). The liver proteins (60 μg per lane) were probed with anti-LBP antibody (Abcam, No. ab25094), anti-CD14 antibody (Abcam, No. ab182032) and β-Actin antibody (Santa Cruz, No. SC-47778). Iii volumes of poly peptide solution were combined with i volume loading buffer. Sixty micrograms of proteins were loaded onto 10% SDS–Page gels. Electrophoresis was performed at sixty V for 2 h at 4°C. Then the proteins were blotted past electrodiffusion for two h at 100 V on nitrocellulose membranes. The membranes were blocked with five% skim milk in T-TBS for ane h at room temperature. The membranes were and so incubated overnight at 4°C with primary antibody in T-TBS containing v% skimmed milk powder. So the membranes were rinsed in T-TBS for v min and repeated for four times. After that, the membranes were incubated for ane h with goat anti-rabbit IgG (H+L) (Thermo Pierce, No. 31160) or goat anti-mouse IgG (H+Fifty) (Thermo Pierce, No. 31210) secondary antibodies, and rinsed in T-TBS for 5 min and repeated five times. Finally, blots were detected using SuperSignal® West Dura Extended Duration Substrate according to the manufacture'due south protocol. The western blot images were analyzed by using the Quantity One software (Biorad).
Statistical Analyses
Linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe) was performed (http://huttenhower.sph.harvard.edu/milky way/) to detect highly-dimensional gut bacteria and characterize the differences between ii or more biological weather condition (or classes; Segata et al., 2011; Zhu et al., 2015). The different features were identified at the OTU and genus levels.
Multivariate analyses were performed with the SIMCA-P software (version 11.5) to discriminate metabolites in colonic contents. Primary component assay (PCA) and partial least squares discriminant analysis (PLS-DA) were performed on the NMR information. PLS-DA models were practical with five-fold cross-validation and evaluated with the R210 and Q2-values. The models were further validated with a permutation exam (200 permutations). In orthogonal projection to latent construction (OPLS) model, X matrix represents for the concentration of all metabolites in each sample, and Y matrix represents for the group information of each sample. It can filter out the racket in information and distinguish the difference betwixt two groups (Trygg and Wold, 2003), and so it was preformed to maximize the separation between ii groups. The metabolites were differentiated on the ground of variable importance in projection (VIP) scores with more than 1 and statistically significant change (t-exam, P < 0.05) was considered to be responsible for the divergence between two groups (Calvani et al., 2014).
Differences in other measurements between whatever 2 groups were evaluated past one-way analysis of variance (1-way ANOVA), and means were compared by Duncan's multiple comparison under the SAS system (version ix.2), p < 0.05 was declared significant.
Results
The Composition of Colonic Polar Metabolites Varied past Diets
A total of 67 dissimilar compounds were identified from all colonic contents based on aneH NMR spectrometry (Supplementary Table 1), including 22 amino acids, 7 short chain fat acids, 8 sugars, 4 phenolic acids, 4 amines, 2 alcohols, 2 amino acid derivatives, 2 ketones, 5 nucleic acid components, 9 other organic acids, one vitamin/cofactor and choline.
Principle component assay revealed great inter- and intra-group variations in metabolites (Figure 1). The craven protein grouping was well-separated from the casein, beef, and soy protein groups, indicating that colonic metabolites showed different responses to craven poly peptide in the diet (P < 0.05) from those to casein, beefiness protein, or soy protein. The soy and beef protein groups showed a not bad similarity.
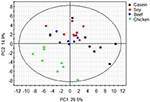
Figure i. PCA scores plot of colonic metabolite profile of rats in response to dissimilar dietary proteins. Each point represents one biological sample.
The tiptop 15 VIP scores of component 1 were listed in Figure 2. The results showed that the rats fed with soy protein had the highest concentrations of propionate, glucose and butyrate. We also establish that the soy protein group had higher levels of short chain fatty acids (923, 779, 666, and 645 μmol/L for the soy, casein, beef, and chicken protein groups, respectively, P < 0.05). The chicken protein group had the highest lactate, but the lowest for the casein group (one,704 vs. 217 μmol/L, respectively, P < 0.05). On the other hand, the casein group had the highest levels of amino acids (leucine, valine, isoleucine), while the craven poly peptide grouping had the everyman levels of these amino acids (P < 0.05).
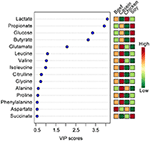
Effigy 2. The tiptop 15 VIP scores of component i. The left part lists significant divergence of metabolites; The middle part shows the acme fifteen VIP scores; The right heatmap shows the concentration of metabolites.
To place the effect of dietary proteins on colonic polar metabolites, pairwise comparing assay was performed using OPLS between the casein group and one of the other 3 groups. One PLS component and one orthogonal component were calculated for all of the models. 1H NMR spectral data were used as the X matrix and classification data was used as the dummy Y matrix. The OPLS plot showed that the overall contour of colonic polar metabolites differed significantly (Figure three). The responsible variables with top 15 VIP scores between the casein and the other three protein groups were showed in Effigy 3. Compared to the casein grouping, the beef poly peptide group had lower levels of glucose, ribose, galactose, butyrate, propionate, uracil, alanine, but higher concentrations of succinate and lactate. The chicken protein group had higher lactate but lower galactose, uracil, butyrate, ribose, propionate, and glucose. The soy protein grouping had college succinate, glucose, propionate, lactate, and butyrate, merely lower leucine, xanthine, valine, uracil, ribose, glutamate, and alanine.
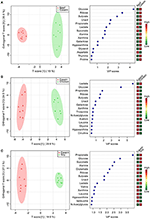
Figure 3. Pairwise comparisons betwixt colonic contents excerpt spectra obtained from the beefiness, craven and soy protein groups using OPLS analysis. Each effigy has two parts: the left part is OPLS score plot, the right part is summit 15 VIP scores. (A) beef protein group vs. casein group; (B) soy protein group vs. casein group; (C) chicken protein group vs. casein group.
Gut Microbiota Had a Singled-out Response to Dietary Proteins
General Information
The 32 colonic samples had a total of 998,150 usable raw reads with an average of 31,192 ± 4,955 reads each (Supplementary Figure 1A). The reads were delineated into 837 operation taxonomy units (OTUs) with an average of 380 ± lxx per sample at a similarity level of 97% (Supplementary Effigy 1B). No meaning difference was observed in reads between any two nutrition groups (p > 0.05), but the beef protein grouping had a greater number of OTUs than the casein and chicken poly peptide groups (p < 0.05). The rarefaction curves did not reach a stable state (Supplementary Figure 1C), just the Shannon–Wiener variety estimates of all samples reached their plateaus (Supplementary Figure 1D), suggesting that the diversity of gut leaner got stable. The Adept'southward coverage index reached an average of 99.73 ± 0.06%, indicating the sequencing methodology was feasible. Ane biological sample in beefiness protein group was observed every bit an outlier as information technology had much smaller number of OTUs and lower Shannon–Wiener diversity estimate than the other samples. And thus the sample was excluded. There were no statistically significant differences (P > 0.05, Supplementary Table ii) among four groups in ACE, Chao, Shannon, Simpson, and Practiced's coverage indices for gut microbiota.
Diet Effect
Principle component analysis revealed great meaning differences in colonic bacteria among diet groups (Figure 4). The chicken protein grouping was well-separated from the casein, beefiness, and soy protein groups in PC 1, while the chicken and beefiness protein groups were separated from the casein and soy protein groups in PC 2. The results indicate that gut bacteria showed different responses to chicken protein in the diet from those to casein, beef protein and soy poly peptide. The soy and casein poly peptide groups showed a great similarity. At phylum level (Effigy v), Firmicutes and Bacteroidetes were the ii most predominant phyla for the four groups, contributing to 83.five, 75.five, 85.6, and 81.ii% of variations the for casein, beef, chicken, and soy protein groups, respectively. Craven protein group had the highest abundance of Bacteroidetes, but the everyman abundance of Firmicutes. Clustering analysis of gut leaner at the phylum level showed that the gut microbiota from the beef, casein, and soy protein groups could be classified into the same subclass which was separated from those of the chicken protein grouping.
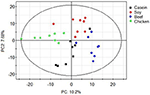
Figure 4. PCA scores plot of gut microbiota of rats in response to different dietary proteins. Each point represents one biological sample.
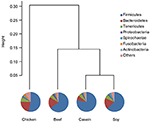
Effigy 5. Relative abundance of gut microbiota at the phylum level. Pie chart showed shows the composition of gut microbiota at the phylum level. Clustering analysis shows the gut microbiota from the beef and soy protein group could be classified into the same subclass and separated from the chicken protein group.
LeFSe analysis was performed on the OTU level to identify specific bacteria for different diet groups. Compared to the casein group, at that place were 96 differential OTUs (Figure vi). Of these OTUs, 16, 12 and 40 OTUs were higher in the beef, chicken and soy poly peptide groups, respectively, and correspondingly, fifteen, 32, and eighteen OTUs were lower in the above three groups, respectively. In particular, the chicken protein group had the highest relative affluence of OTUs for genus Lactobacillus (OTU427 and OTU746), while the soy protein group had the highest relative abundance of OTUs for family unit Ruminococcaceae.
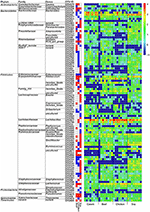
Figure half-dozen. Gut leaner at the OTU level in response to dietary proteins using LefSe. (1) The left function lists pregnant departure of OTU and corresponding phyla, families and genera; (2) The eye heatmap shows rich group and poor group of each OTU; (3) The correct heatmap shows the relative abundance of OTU (log 10 transformed). Each column represents one biological sample and each row represents ane OTU.
Dietary Proteins Affects the Gut-Derived Endotoxins Level in Liver
LPS, gut-derived endotoxins, can demark to LBP in liver and activate Kupffer cells via CD14 receptor. Pro-inflammatory cytokines are released and this is postulated to promote liver injury.
No meaning difference was found in LBP mRNA level among dietary groups (P > 0.05, Figure 7A). Nevertheless, the levels of CD14 mRNA were establish to be significantly lower in the casein, beef, and craven protein groups than the soy protein group (P < 0.001, Figure 7B). The profile of LBP and CD14 proteins expression were detected by Western blotting. The results indicated that the LBP and CD14 protein levels were significantly higher in the soy protein grouping than any other protein group (P < 0.05, Figures 8A–C). The expression of LBP protein level was significantly lower in craven protein group than in casein and beef poly peptide groups (P < 0.05, Figures 8A,B), while no pregnant divergence in CD14 protein level was observed between any 2 of casein, beef, and craven protein groups (P > 0.05, Figure 8).
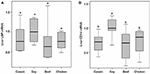
Effigy vii. Cistron expression levels of LBP (A) and CD14 (B) in the liver. All mRNA quantification data were normalized to the housekeeping gene β-actin. Gene expression levels were expressed as values relative to the soy poly peptide grouping. Ways with different superscripts differed significantly (P < 0.05).
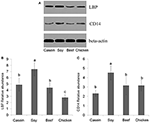
Effigy 8. Western blotting profiles of LBP and CD14. (A) western blotting results; (B) LBP relative abundance; (C) CD14 relative abundance. a,b,c, Means with dissimilar alphabetic character differed significantly (P < 0.05).
Give-and-take
Gut microbiota plays a crucial role in homo diet and health. Food components are ordinarily digested in the stomach and small intestine, but the indigestible nutrient compounds and endogenous proteins would enter into the large intestine for microbial fermentation and putrefaction, which shape diverse gut microbiota and metabolites (Van Hylckama Vlieg et al., 2011; Ridaura et al., 2013; Rist et al., 2013). The present study showed a significant impact of dietary proteins on gut microbiota and metabolites in rats.
Firmicutes and Bacteroidetes were observed to be the prevalent phyla in all samples. The casein, beef and soy protein groups had lower ratios of Firmicutes to Bacteroidetes (F/B ratio) than the craven protein group (Supplementary Figure 3). The F/B ratio in the gut has been shown to exist associated with obesity for both human and model animals (Turnbaugh et al., 2006, 2008, 2009; Turnbaugh and Gordon, 2009). Yet, all animals in the nowadays study did not show obese characteristics. An interesting ascertainment was that the torso weight of one rat in beefiness protein group showed significant decrease during the terminal 2 weeks of feeding (Lin et al., 2016). The F/B ratio was the lowest (0.xi vs. 4.viii% for the average of other seven rats in beef poly peptide group; Supplementary Data 1).
Compared to the casein grouping, rats fed with chicken protein had higher level of beneficial genus Lactobacillus, while the soy protein group had the everyman abundance of this bacterium. Lactobacillus has been considered as a key player in host metabolic balance (Zhang et al., 2009; Arora et al., 2012). Higher abundance of Lactobacillus may reduce the antigen load from gut leaner to the host, and alleviate inflammation responses and metabolic syndromes (Cani et al., 2007; Zhao, 2013). LBP could exist every bit a biomarker of host inflammatory response and antigen load in blood (Zweigner et al., 2006). Our previous data showed that intake of meat proteins reduced serum LBP level compared to intake of soy poly peptide (Zhu et al., 2015). In the present study, the chicken protein group had the highest concentration of lactate, which is in accordance with the changes of Lactobacillus affluence. The beingness of Lactobacillus helps to maintain a high level of lactic acrid, which would promote the lactate-utilizing species to thrive (Ruth et al., 2006; Chaucheyras-Durand and Duran, 2009). Western blotting results showed that LBP and CD14 were up-regulated in the rats fed with soy protein, equally compared to the casein diet group. The chicken and beef protein diet did non take such an effect. In addition, the protein levels of glutathione South-transferases (GSTs), which involve detoxification or defence responses (Kim et al., 2004), were two-fold higher in the soy poly peptide group than the other two groups (Supplementary Figure 2), which was in accordance with the results of LBP mRNA and CD14 mRNA in the liver (Zhu et al., 2015). LBP is an acute phase protein, and pro-inflammatory cytokines can increment LBP level (Lukkari et al., 1999). Our results suggested that intake of chicken protein at a normal dose may exist more beneficial for the proliferation of commensal bacteria, every bit compared to the soy protein group.
Amino acid concentrations in colonic contents were much college for the casein and beef protein groups than those for the chicken and soy protein groups. This could be attributed to several aspects. Firstly, some gut bacteria have the capacity of utilizing undigested dietary proteins and producing amino acids by excreting proteolytic enzymes. These bacteria belong to Clostridium, Fusobacterium, and Acidaminococcus. Equally shown in a higher place, genus Fusobacterium was college in the casein and beef protein groups than the other two groups. Secondly, the composition of dietary proteins could also accept a certain influence on microbial fermentation and the levels of amino acids in gut (Nocek et al., 2002; Nocek and Kautz, 2006). We also monitored the levels of amino acids in claret, and plant that the rank of the levels of all amino acids was chicken protein grouping > soy poly peptide group > beef protein group (Xuebin Shi, personal advice). This upshot showed that craven protein may be easier to digest and blot in the small intestine than soy and beef proteins, which caused few amino acids to enter into the big intestine (Christensen, 1984). Thirdly, the absorption activity of gut epithelium might have an impact on the levels of amino acids (Zhao et al., 2011). Therefore, beef and soy proteins could exist less digested and absorbed in the small intestine and modify the limerick of gut bacteria in the big intestine, which results in higher levels of amino acids in colonic contents. The underlying machinery needs further investigations.
The intake of meat proteins and casein could reduce the fermentation of non-digestive fibers in rat colon. This was reflected by lower levels of short-concatenation fatty acids, glucose, N-acetylglucosamine, galactose, and ribose in colonic contents for the casein, beef, and craven protein groups. Cornstarch and dextrinized cornstarch in diets were rich in dietary fibers and glycans which could not be degraded in both breadbasket and pocket-sized intestine considering of lacking specific enzymes in the host (Gill et al., 2006). However, there were at least 81 dissimilar glycoside hydrolase families in gut leaner that contain genes involved in starch and sucrose metabolism, and the metabolism of glucose, galactose, fructose, arabinose, mannose, and xylose (Nocek et al., 2002). Previous studies showed that resistant starch diets could increase the abundance of Ruminococcaceae phylotypes in the gut (Walker et al., 2010; Salonen et al., 2014). Our results also showed that soy protein group had higher relative abundance of Ruminococcaceae (OTU 584, OTU682, OTU724, OTU779) that had a positive correlation with the levels of glucose, galactose, and ribose. This indicates that the intake of soy protein may favor the colonization of gut bacteria that take the capacity of degrading glycans. In addition, gut bacteria can apply undigested carbohydrates, proteins, peptides, and amino acids to produce brusque-chain fatty acids that are energy source (especially butyrate) for colonocytes (Nicholson et al., 2012). Brusque-concatenation fatty acids have been shown to be associated with health benefits including glucose homeostasis, lipid metabolism, and reduced colon cancer run a risk (Byrne et al., 2015). The levels of butyrate and monosaccharide were associated positively with the relative abundances of Alistipes, Prevotella, Alloprevotella, and Oscillibacter (Zhao et al., 2013). Prevotella has the capability of utilizing a wide range of substrates and is a critical propionate producer (Reichardt et al., 2014). Although, the VIP score of propionate was <ane, the level of propionate was higher in the soy protein group than those of the casein, beefiness, and chicken poly peptide groups (141, 106, 85, and 58 μmol/50 for the soy, casein, beef, and chicken protein groups, respectively). Gut microbiota can help the host become more energy from foods by fermenting undigested food ingredients and endogenous proteins to produce SCFAs (Cummings et al., 1987; Wong et al., 2006). This may explain the phenomenon that the soy protein group had lower body weight and weight gain, but higher visceral fat content (Zhu et al., 2015).
Based on the above results, soy protein intake could induce more carbohydrate metabolism as compared to beefiness and chicken proteins. Most of the amino acids (AAs) and their related metabolites were more arable in casein and beefiness protein groups. The effluvious amino acids, such equally phenylalanine and branched-chain amino acids, including valine, leucine, and isoleucine, were too higher in casein poly peptide grouping. This could be attributed to college affluence of gut leaner such equally Fusobacterium, which has the capacity of utilizing undigested dietary proteins and producing amino acids by excreting proteolytic enzymes.
Determination
The type of proteins in diets had a meaning impact on the compositions of gut bacteria and metabolites. Chicken poly peptide promoted the growth of genus Lactobacillus, while soy protein promoted the growth of family Ruminococcaceae. Compared to meat proteins, the intake of soy protein may increase the degradation of dietary fibers and glycans and produce higher levels of short chain fatty acids. The casein and beefiness protein groups had higher levels of amino acids than the chicken protein grouping. Although the soy protein group had higher levels of SCFAs, the relative affluence of beneficial bacteria was lower, and the detoxification or defense responses related proteins in host liver were higher than meat protein groups. Meanwhile, long-term intake of soy poly peptide led to the up-regulation of CD14 and LBP in liver and the level of LBP in serum (The consequence about LBP in serum was shown in reference 24, our previously published paper), suggesting that bacterial endotoxins were elevated. Our results confirmed that long-term intake of meat proteins can maintain a more than balanced composition of gut bacteria and reduce the antigen load and inflammatory response from gut bacteria to the host.
Additional Information
Sequence information: all sequence data take been deposited in the NCBI Sequence Read Archive nether accretion lawmaking SRP066996.
Author Contributions
The seven authors are justifiably credited with authorship, according to the authorship criteria. YZ: Design, conquering of data, assay, and estimation of data, drafting the manuscript, final approval given; CL: Conception, design, fractional conquering of data, analysis, and estimation of information, drafting the manuscript, funding holder, final approval given; 40: Data acquisition of animal rearing, final approval given; XX: Critical revision of manuscript, final approval given; XS: Data analysis, final blessing given; KY: Acquisition of data, final approval given; GZ: Conception, pattern, assay and interpretation of information, critical revision of the manuscript, final approval given.
Funding
This piece of work was funded past grants 31530054 (National Natural Science Foundation of People's republic of china, NSFC) and 31471600 (National Natural Science Foundation of Prc, NSFC).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of whatever commercial or financial relationships that could be construed equally a potential conflict of interest.
Acknowledgments
Special thanks were given to Weihua Chen, Mengjie Li, Qiayu Wu, Siying Wen, Li Li, Hedong Lu, and Huixing Lin from Nanjing Agronomical Academy for their help during creature feeding and sampling.
Supplementary Material
The Supplementary Textile for this commodity tin can exist found online at: https://world wide web.frontiersin.org/article/ten.3389/fmicb.2017.01395/total#supplementary-material
Supplementary Figure one. Diversity estimation of ceacel microbiota in all samples. (A) The average number of usable raw reads (mean and standard deviation). I-way ANOVA and Duncan'southward multiple comparisons indicated that pork protein groups had higher affluence of usable raw reads than soy and chicken protein groups (p < 0.05); (B) The average number of OTU (hateful and standard difference). 1-way ANOVA indicated that in that location was no significant difference between any two nutrition groups in OTU number (p > 0.05); (C) Rarefaction curves. Each line represents one rat; (D) Shannon–Wiener diverseness index curves. Each line represents one rat.
Supplementary Figure 2. The expression of glutathione S-transferases in liver.
Supplementary Figure three. The F/B ratio of rats fed different kinds of dietary proteins. a,b, Means with different letter differed significantly (P < 0.05).
Supplementary Table ane. The composition of metabolites contour.
Supplementary Table 2. Richness and variety indexes relative to each sample.
References
Arora, T., Anastasovska, J., Gibson, G., Tuohy, Thousand., Sharma, R., Bell, J., et al. (2012). Consequence of Lactobacillus acidophilus NCDC 13 supplementation on the progression of obesity in nutrition-induced obese mice. Br. J. Nutr. 108, 1382–1389. doi: 10.1017/S0007114511006957
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bastide, North., Chenni, F., Audebert, 1000., Santarelli, R., Taché, South., Naud, N., et al. (2015). A cardinal role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 75, 870–879. doi: ten.1158/0008-5472.Tin can-14-2554
PubMed Abstract | CrossRef Full Text | Google Scholar
Byrne, C. S., Chambers, E. S., Morrison, D. J., and Frost, Yard. (2015). The role of brusk concatenation fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 39, 1331–1338. doi: 10.1038/ijo.2015.84
PubMed Abstract | CrossRef Total Text | Google Scholar
Calvani, R., Brasili, Due east., Praticò, Grand., Capuani, G., Tomassini, A., Marini, F., et al. (2014). Fecal and urinary NMR-based metabolomics unveil an aging signature in mice. Exp. Gerontol. 49, 5–eleven. doi: 10.1016/j.exger.2013.ten.010
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, 1000., Knauf, C., Bastelica, D., et al. (2007). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. doi: 10.2337/db06-1491
PubMed Abstract | CrossRef Full Text | Google Scholar
Cummings, J. (ed.). (1997). "Carbohydrate and protein digestion: the substrates available for fermentation," in The Big Intestine in Nutrition and Illness (Brussels: Institut Danone), 15–41.
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P., and MacFarlane, G. T. (1987). Curt concatenation fatty acids in homo big intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. doi: 10.1136/gut.28.x.1221
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cummings, J., and MacFarlane, G. (1991). The command and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70, 443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
David, L. A., Maurice, C. F., Carmody, R. Northward., Gootenberg, D. B., Button, J. E., Wolfe, B. Due east., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: x.1038/nature12820
PubMed Abstract | CrossRef Full Text | Google Scholar
Twenty-four hour period, 50. (2013). Proteins from land plants–potential resources for human nutrition and food security. Trends Food Sci. Technol. 32, 25–42. doi: 10.1016/j.tifs.2013.05.005
CrossRef Total Text | Google Scholar
Flintstone, H. J., Duncan, Southward. H., Scott, 1000. P., and Louis, P. (2015). Links between nutrition, gut microbiota composition and gut metabolism. P. Nutr. Soc. 74, thirteen–22. doi: 10.1017/S0029665114001463
PubMed Abstract | CrossRef Full Text | Google Scholar
Geypens, B., Claus, D., Evenepoel, P., Hiele, Thousand., Maes, B., Peeters, M., et al. (1997). Influence of dietary poly peptide supplements on the formation of bacterial metabolites in the colon. Gut 41, lxx–76. doi: ten.1136/gut.41.1.lxx
PubMed Abstract | CrossRef Full Text | Google Scholar
Gill, S. R., Pop, M., DeBoy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. Southward., et al. (2006). Metagenomic analysis of the human distal gut microbiome. Scientific discipline 312, 1355–1359. doi: x.1126/science.1124234
PubMed Abstract | CrossRef Total Text | Google Scholar
Hamer, H. Chiliad., De Preter, V., Windey, K., and Verbeke, Thou. (2012). Functional analysis of colonic bacterial metabolism: relevant to health? Am. J. Physiol-Gastr. L. 302, G1–G9. doi: 10.1152/ajpgi.00048.2011
PubMed Abstract | CrossRef Full Text | Google Scholar
Järveläinen, H. A., Oinonen, T., and Lindros, M. O. (1997). Alcohol-Induced Expression of the CD14 Endotoxin Receptor Poly peptide in Rat Kupffer Cells. Alcohol Clin. Exp. Res. 21, 1547–1551. doi: 10.1111/j.1530-0277.1997.tb04488.x
PubMed Abstruse | CrossRef Total Text | Google Scholar
Kim, S., Sohnb, I., Ahna, J., Leea, K., Leec, Y. S., Lee, Y. S., et al. (2004). Hepatic factor expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 340, 99–109. doi: 10.1016/j.gene.2004.06.015
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ley, R. Eastward., Peterson, D. A., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the homo intestine. Prison cell 124, 837–848. doi: x.1016/j.jail cell.2006.02.017
PubMed Abstract | CrossRef Full Text | Google Scholar
Lin, Ten., Li, Y., Zhu, Y., Shi, 10., Zhou, G., Xu, X., et al. (2016). Effect of dietary pork and beef proteins on physiological responses of growing rats. Food Sci. 37, 175–179. doi: 10.7506/spkx1002-6630-201605031
CrossRef Full Text
Lukkari, T. A., Järveläinen, H. A., Oinonen, T., Kettunen, E., and Lindros, 1000. O. (1999). Short-term ethanol exposure increases the expression of Kupffer jail cell CD14 receptor and lipopolysaccharide binding poly peptide in rat liver. Alcohol Booze. 34, 311–319. doi: ten.1093/alcalc/34.three.311
PubMed Abstract | CrossRef Full Text | Google Scholar
Muegge, B. D., Kuczynski, J., Knights, D., Clemente, J. C., González, A., Fontana, 50., et al. (2011). Diet drives convergence in gut microbiome functions across mammalian phylogeny and inside humans. Science 332, 970–974. doi: 10.1126/scientific discipline.1198719
PubMed Abstract | CrossRef Total Text | Google Scholar
Nicholson, J. K., Holmes, E., Kinross, J., Burcelin, R., Gibson, Thousand., Jia, W., et al. (2012). Host-gut microbiota metabolic interactions. Science 336, 1262–1267. doi: x.1126/scientific discipline.1223813
PubMed Abstruse | CrossRef Total Text | Google Scholar
Nocek, J. E., and Kautz, W. P. (2006). Straight-fed microbial supplementation on ruminal digestion, health, and performance of pre- and postpartum dairy cattle. J. Dairy. Sci. 89, 260–266. doi: 10.3168/jds.S0022-0302(06)72090-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Nocek, J. E., Kautz, W. P., Leedle, J. A., and Allman, J. G. (2002). Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 85, 429–433. doi: 10.3168/jds.S0022-0302(02)74091-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Pan, A., Lord's day, Q., Bernstein, A. One thousand., Schulze, M. B., Manson, J. E., Stampfer, One thousand. J., et al. (2012). Red meat consumption and mortality: results from 2 prospective cohort studies. Arch. Intern. Med. 172, 555–563. doi: 10.1001/archinternmed.2011.2287
PubMed Abstract | CrossRef Full Text | Google Scholar
Reichardt, N., Duncan, S. H., Young, P., Belenguer, A., Leitch, C. Thou., Scott, K. P., et al. (2014). Phylogenetic distribution of iii pathways for propionate product within the man gut microbiota. ISME J. viii, 1323–1335. doi: 10.1038/ismej.2014.fourteen
PubMed Abstract | CrossRef Full Text | Google Scholar
Ridaura, 5. One thousand., Faith, J. J., Rey, F. E., Cheng, J., Duncan, A. E., Kau, A. L., et al. (2013). Gut microbiota from twins discordant for obesity attune metabolism in mice. Science 341, 1069–1070. doi: 10.1126/science.1241214
PubMed Abstract | CrossRef Full Text
Rist, V. T., Weiss, E., Eklund, M., and Mosenthin, R. (2013). Impact of dietary protein on microbiota composition and action in the gastrointestinal tract of piglets in relation to gut wellness: a review. Animal 7, 1067–1078. doi: 10.1017/S1751731113000062
PubMed Abstract | CrossRef Full Text | Google Scholar
Rist, 5. T., Weiss, Eastward., Sauer, N., Mosenthin, R., and Eklund, M. (2014). Upshot of dietary protein supply originating from soybean meal or casein on the abdominal microbiota of piglets. Anaerobe 25, 72–79. doi: x.1016/j.anaerobe.2013.10.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Ruth, E. L., Daniel, A. P., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Jail cell 124, 837–848. doi: x.1016/j.jail cell.2006.02.017
CrossRef Full Text | Google Scholar
Salonen, A., Lahti, L., Salojärvi, J., Holtrop, K., Korpela, Chiliad., Duncan, S. H., et al. (2014). Impact of nutrition and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 8, 2218–2230. doi: ten.1038/ismej.2014.63
PubMed Abstract | CrossRef Full Text | Google Scholar
Scott, K. P., Gratz, S. W., Sheridan, P. O., Flintstone, H. J., and Duncan, S. H. (2013). The influence of diet on the gut microbiota. Pharmacol. Res. 69, 52–sixty. doi: 10.1016/j.phrs.2012.x.020
PubMed Abstract | CrossRef Full Text | Google Scholar
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. South., et al. (2011). Metagenomic biomarker discovery and caption. Genome Biol. 12:R60. doi: x.1186/gb-2011-12-6-r60
PubMed Abstruse | CrossRef Full Text | Google Scholar
Subramanian, Southward., Huq, S., Yatsunenko, T., Haque, R., Mahfuz, Grand., Alam, M. A., et al. (2014). Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421. doi: 10.1038/nature13421
PubMed Abstract | CrossRef Full Text | Google Scholar
Tang, W. Due west., Wang, Z., Levison, B. South., Koeth, R. A., Britt, E. B., Fu, 10., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584. doi: x.1056/NEJMoa1109400
PubMed Abstract | CrossRef Full Text | Google Scholar
Trygg, J., and Wold, S. (2003). O2-PLS, a ii-block (X-Y) latent variable regression (LVR) method with an integral OSC filter. J. Chemometr. 17, 53–64. doi: ten.1002/cem.775
CrossRef Full Text | Google Scholar
Turnbaugh, P. J., Bäckhed, F., Fulton, Fifty., and Gordon, J. I. (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Jail cell Host. Microbe 3, 213–223. doi: 10.1016/j.chom.2008.02.015
PubMed Abstract | CrossRef Full Text | Google Scholar
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540
PubMed Abstruse | CrossRef Total Text | Google Scholar
Turnbaugh, P. J., Ley, R. East., Mahowald, M. A., Magrini, 5., Mardis, Eastward. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
PubMed Abstract | CrossRef Full Text | Google Scholar
Tyakht, A. V., Kostryukova, E. South., Popenko, A. Southward., Belenikin, M. South., Pavlenko, A. V., Larin, A. K., et al. (2012). Man gut microbiome viewed across age and geography. Nature 486, 222–227. doi: ten.1038/nature11053
CrossRef Total Text | Google Scholar
Van Hylckama Vlieg, J. E., Veiga, P., Zhang, C., Derrien, M., and Zhao, 50. (2011). Impact of microbial transformation of food on health—from fermented foods to fermentation in the gastro-intestinal tract. Curr. Opin. Biotechnol. 22, 211–219. doi: 10.1016/j.copbio.2010.12.004
PubMed Abstract | CrossRef Total Text | Google Scholar
Walker, A. W., Ince, J., Duncan, South. H., Webster, L. M., Holtrop, G., Ze, X., et al. (2010). Ascendant and nutrition-responsive groups of bacteria within the human colonic microbiota. ISME J. v, 220–230. doi: 10.1038/ismej.2010.118
PubMed Abstract | CrossRef Total Text | Google Scholar
Weiss, J. (2003). Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defense force against Gram-negative bacteria. Biochem. Soc. Trans. 31, 785–790. doi: 10.1042/bst0310785
PubMed Abstract | CrossRef Full Text | Google Scholar
Wong, J. Grand. W., de Souza, R., Kendall, C. Westward. C., Emam, A., and Jenkins, D. J. A. (2006). Colonic health: fermentation and brusque chain fat acids[J]. J Clin Gastroenterol. 40, 235–243. doi: 10.1097/00004836-200603000-00015
PubMed Abstruse | CrossRef Full Text | Google Scholar
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y. Y., Keilbaugh, S. A., et al. (2011). Linking long-termdietary patterns with gut microbial enterotypes. Science 334, 105–108. doi: 10.1126/science.1208344
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhang, C., Zhang, M., Wang, S., Han, R., Cao, Y., Hua, W., et al. (2009). Interactions betwixt gut microbiota, host genetics and nutrition relevant to development of metabolic syndromes in mice. ISME J. iv, 232–241. doi: x.1038/ismej.2009.112
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhao, L., Wu, G., and Zhu, Due west. (2011). Amino acid metabolism in abdominal bacteria: links between gut ecology and host health. Front end Biosci. sixteen, 1768–1786. doi: 10.2741/3820
CrossRef Full Text | Google Scholar
Zhao, Y., Wu, J., Li, J., Zhou, N., Tang, H., and Wang, Y. (2013). Gut microbiota composition modifies fecal metabolic profiles in mice. J Proteome Res. 12, 2987–2999. doi: 10.1021/pr400263n
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhu, Y., Lin, X., Zhao, F., Shi, 10., Li, H., Li, Y., et al. (2015). Meat, dairy and plant proteins alter bacterial limerick of rat gut leaner. Sci. Rep. v:15220. doi: x.1038/srep15220
CrossRef Full Text | Google Scholar
Zweigner, J., Schumann, R. R., and Weber, J. R. (2006). The role of lipopolysaccharide-binding protein in modulating the innate allowed response. Microbes Infect. 8, 946–952. doi: x.1016/j.micinf.2005.x.006
PubMed Abstruse | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fmicb.2017.01395/full
0 Response to "Protien in Soy Protien in Beef"
Post a Comment